Article Contents
| Clin Exp Pediatr > Volume 65(9); 2022 |
|
Abstract
Background
Plastic bottles are widely used by people to feed their infants when breastfeeding is not possible. Bisphenol A (BPA), an endocrine disruptor is widely used in the manufacturing of plastic wares and is leached out from these plastic wares on exposure to high temperature, changed pH, or cleaning the plastic wares by harsh detergents.
Purpose
Feeding through plastic bottles over prolong duration is expected to expose the infants to leached BPA. Hence the present study was taken up to compare the effects of breastfeeding and plastic bottle feeding on biochemical parameters in infants and also detect for the presence of free BPA or its metabolite in the infants.
Methods
Biochemical tests like lipid profile, liver function tests, creatine-kinase–MB (CK-MB), serum urea, serum electrolytes were performed on blood samples obtained from infants who were breastfed and plastic bottle fed. Further, plasma and urine samples of the infants were subjected to Liquid chromatography-mass spectrometry analysis for detecting free BPA and BPA glucuronide.
Results
Biochemical changes in form of raised triglycerides, cholesterol, low-density lipoproteins, very low-density lipoproteins and increase in CK-MB, serum urea were observed in plastic bottle fed infants. BPA glucuronide was also detected in the urine of these infants. Free BPA was not detected in plasma or urine samples of the infants except in one plasma sample from bottle-fed group.
Graphical abstract
Breastfeeding has been always considered to be extremely beneficial for infants since ages and emphasis is laid to promote breastfeeding for promoting both infant’s and mother’s health. However, in certain situations, breastfeeding is not possible like the death of a mother, inability to produce enough milk due to various reasons, improper feeding techniques so that infant is unable to feed properly, adopted infants, a mother suffering from a certain disease, etc. In such situations, infants have to be put on top feeding through bottles. The feeding bottles available in the market are made of either glass or plastics. Most people prefer to use plastic feeding bottles as it is easily available, cost-effective, non-breakable and maintenance is less cumbersome as compared to its glass counterpart. These bottles are manufactured using type 7 plastics [1]. The type 7 plastics are reported to leach out bisphenol A (BPA) when they are exposed to high temperatures, acidic pH or if cleansed or scrubbed using harsh detergents [2]. Hence, repeated cleaning of these bottles leads to more leaching of BPA.
BPA, a known endocrine disruptor acts through estrogen receptors [3,4]. Studies have reported that BPA produces reproductive toxicity which manifests as a decrease in fertility in female mice, and decreased fertility in male rats [5-12]. In experimental animals, BPA has also been shown to produce neural and behavioral alterations [13-16]. Studies have revealed the association between prenatal exposure of BPA and childhood respiratory and allergic diseases [17]. Further BPA is reported to possess carcinogenic effects and produce hyperplasia of ducts of mammary glands, prostate, etc [18,19]. Association of BPA exposure with cardiac diseases, hepatic defects, and childhood obesity has been documented in several studies [20-22]. Recently, BPA leached from plastic infant feeding bottles have shown to produce oxidative stress-induced tissue damage in rats [23].
The main route of exposure of BPA in humans is through diet. Following ingestion, conjugation of BPA takes place in the liver which forms water-soluble BPA glucuronide and sulphate. These water-soluble metabolites are excreted through the kidneys. However, not all BPA is conjugated and some remains as unconjugated BPA (free form). The free BPA is water- insoluble and therefore not excreted by the kidneys [24]. The unconjugated BPA remains inside the body tissues and is said to be biologically active. BPA has been reported to be detected in the urine of adult population in the United States [25]. Studies have shown that consumption of fluids through plastic bottles increases the load of BPA in urine [26]. Infants have a lower availability of conjugating enzymes [27]. This is expected to cause less conjugation and more accumulation of free BPA levels in the tissues altering their functioning.
Use of BPA in food containers and infant bottles has been banned by many countries [28]. However, in many developing countries, the measures to check the use of BPA in plastic wares is not adequate and the chemical is still used to manufacture plastic wares including infant feeding bottles. Further, BPA free plastic wares which are available in market, have been reported to possess endocrine disruptors other than BPA which also act through the estrogen receptors [29,30]. This raises the concern while feeding the infants through such products.
The feeding practices in case of infants being fed through plastic bottles need a deeper insight since people adopt certain practices of using these bottles. For example, before use, the infant feeding bottles are washed using detergent and brush bottle cleaner. After detergent wash, the bottles are washed under tap water. Thereafter, these washed bottles are soaked into hot water for sterilization and allowed to dry. Once dried, these bottles are ready to use. Usually, the bottles are filled with lukewarm or warm milk or water or top feed supplements feed the infants. Further, the bottles are placed in bottle warmers in case, the contents of the bottles turn cold. This entire process of cleaning and filling up the bottles with lukewarm/warm milk/water and further rewarming them in bottle warmers increases the leaching of BPA through the plastic bottles [23]. The leaching is more prominent in the case of old bottles. Hence, we hypothesized that BPA leached from plastic feeding bottles would possibly alter physiological mechanisms in the infants, as BPA is a proven endocrine-disrupting agent.
With this background knowledge, we wanted to study whether feeding through plastic bottle produces any change in biochemical parameters in human infants. Further, we wanted to explore whether BPA (free and metabolite) is present in plasma and urine of these infants since there was no prior data available for BPA levels in infants of the region included in the study.
The present study was undertaken after obtaining clearance from the institutional ethical committee (Letter No 200/GMC/IEC/03/2014). A prior informed written consent from the parents/guardians of the infants was obtained at the beginning of the study. Infants suffering from any systemic disease were excluded from the study. Infants who were fed through glass feeding bottles and neonates were not included in the present study. The subjects were recruited from Feb 2016 to June 2017. The infants who were brought to Pediatrics Out Patient Department (OPD) and selected for the study belonged to the Kumaon region of Uttarakhand, India and were divided into 2 groups: group 1 (control group; n=18): This group comprised of healthy infants who were on breast milk and not fed by plastic bottles; group 2 (study group n=45): This group comprised of healthy infants who did not receive breast milk and were on top feed administered through plastic bottles.
A detailed history was obtained from parents/guardians of these infants regarding their feeding practices, as to whether they were on breast milk or on top feed fed through plastic feeding bottles. Further, they were also asked about the type of plastic bottle which they used for feeding the infants. According to the parents, the bottles were purchased from local shops. They were unaware about any specific brand or type of plastic. They did not have any information about BPA and were ignorant about it. They informed that they would wash the bottles with utensil cleaning detergents and would dip them in boiled water for sterilization before use. They filled these bottles with lukewarm milk/water to feed their infants. The top feed comprised of mixed practice of feeding bovine milk, goat milk, water, and formula feed. Thereafter, blood and urine were collected from infants in both groups after obtaining consent from parents/guardians. The samples were collected in the morning hours during OPD time. Infants are fed on demand every 2–2.5 hours, hence care was taken that the duration between last feed taken and collection of samples was minimum 2 hours. Blood (2 mL) was collected in ethylene diamine tetra acetic acid and plain vial for each infant and urine (4 mL) was collected in sterile urine container. Ninety-three blood samples and 44 urine samples were collected altogether in both the groups. Of these, 30 blood samples were discarded due to hemolysis or insufficient quantity (Fig. 1). The rest of the blood samples were centrifuged to separate plasma and serum from the whole blood. The plasma and urine of these infants were used for estimation of BPA. The serum samples obtained from the infants were subjected to biochemical analysis for the study of various biochemical parameters.
Briefly, all analytes were estimated using full automated clinical chemistry analyzer AW480. Total and direct bilirubin levels were estimated by Beckman Coulter and modified Vandenberg’s method. Indirect bilirubin was calculated from the direct and total bilirubin values obtained from the aforesaid methods. Bowers and McComb method were used for estimation of alkaline phosphatase. Kinetic method/International Federation of Clinical Chemistry recommended method was used to estimate serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic-pyruvic transaminase (SGPT). Cholesterol oxidase/peroxidase amino phenazone was used to measure total cholesterol. Glycerine phosphate oxidase peroxidase (GPO/PAP) was used for estimation of triglycerides. Low-density lipoprotein (LDL) and high-density lipoprotein (HDL) were estimated using 2 reagent homogenous system. Very low-density lipoprotein (VLDL) was calculated from the values obtained for total cholesterol, LDL, HDL using Freidwalds equation. Serum urea was tested by Colorimetric urease test method, creatinekinase–MB (CK-MB) levels were measured by immunoinhibition method.
High performance liquid chromatography (HPLC) was used to detect presence of BPA or BPA β D-Glucuronide (BPA-G) in the samples. The system used was Shimadzu Liquid Chromatography System (Shimadzu Corp., Kyoto, Japan, Model SPD-10A, LC10AT) comprising of double plunger pump; rheodyne injector with 20-µL loop; ultraviolet-visible spectroscopy detector. Separation of BPA/BPA-G was done by using C18 reverse phase column (Lichrospher 100 RP-18.5 µm [125 mm×4 mm]). The standards for BPA were made by dissolving 2 mg of pure BPA/BPA-G (Sigma-Aldrich, St. Louis, MO, USA) in 2 mL of acetonitrile (ACN) from which the concentrations of 10, 5, 2.0, 1, 0.5, 0.2, 0.1, 0.05, 0.025, 0.01 μg/mL were made. Twenty microliter of these concentrations was injected into HPLC. The mobile phase consisted of water: ACN (30: 70, v/v) with and pH adjusted to 4.3 with 0.1N HCl, using an isocratic form with a flow rate of 0.5 mL/min. The detector wavelength was set at 277 nm. The limit of quantification was 0.01 µg/mL.
To further confirm for the presence of BPA or BPA-G, random samples of plasma and urine were subjected to liquid chromatography-mass spectrometry (LCMS/MS). LCMS/MS is known to have more accuracy, better sensitivity and specificity as compared to HPLC alone, hence few samples were subjected to LCMS/MS for qualitative analysis of free BPA and BPA glucuronide in addition to HPLC. Ultraperformance liquid chromatography (UPLC-H class) coupled with quadrupole time-of-flight was used for LCMS/MS. Mobile phase was of 0.1% formaldehyde (FA) and 2% ACN was mixed to prepare aqueous solution. 98% ACN, 2% of 0.1% FA was used to prepare organic solution. The ethylene bridged hybrid-C18 (1.7 µm) column was used for the process.
Both male and female infants 7.6±3 months (1.5–12 months) weighing 8.03±2.1 kg (4–9 kg) were included in the study after obtaining written consent from their parents. The bottle-fed infants who were chosen for the study did not receive breast milk. They were exposed to bottle feeding since their birth. Following biochemical changes were observed.
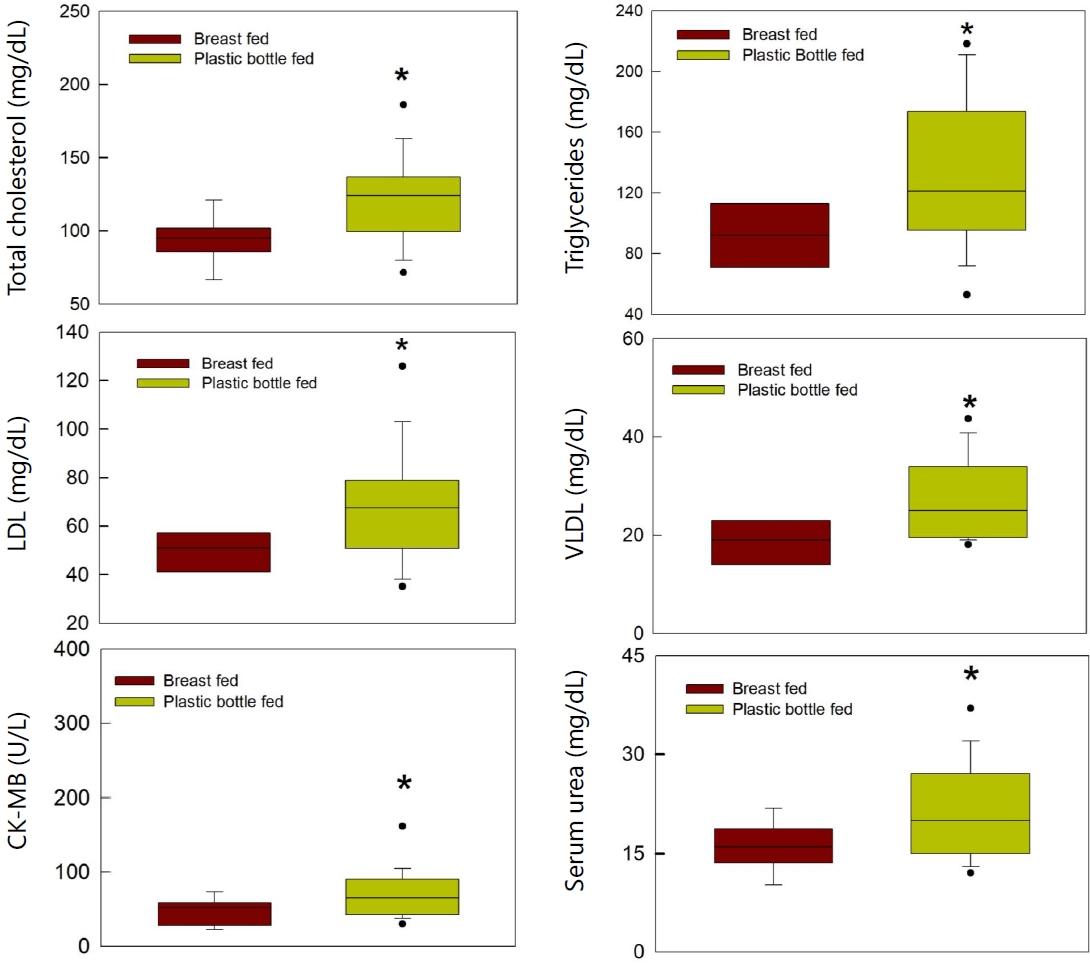
Biochemical parameters were examined in both groups of infants. CK-MB levels were significantly high in bottle-fed infants as compared to breastfed group (P=0.02). The urea levels were also significantly high in bottle-fed infants (P=0.038). Total cholesterol, triglycerides, VLDL, and LDL levels were significantly high in bottle-fed infants compared to breastfed infants of respective groups (P=0.008, P=0.039, P=0.019, P=0.038 respectively) (Fig. 2). The creatinine levels were not different in breastfed or bottle-fed infants. The total bilirubin levels were within the normal limits in both groups. Both SGOT and SGPT levels were not different in both groups. Serum Sodium and potassium were also within normal limits in all the infants of both the groups.
Free BPA could not be detected by HPLC in the plasma and urine samples analyzed. Hence 24 urine and 20 plasma random samples were subjected to LCMS/MS for the presence of free BPA and BPA glucuronide
Twelve urine and 10 plasma random samples from both groups (6 urine samples and 5 plasma samples in each group) were analyzed by LCMS/MS for the presence of free BPA. Only one plasma sample of a bottle-fed infant tested positive for free BPA by LCMS/MS. None of the urine samples tested positive for free BPA.
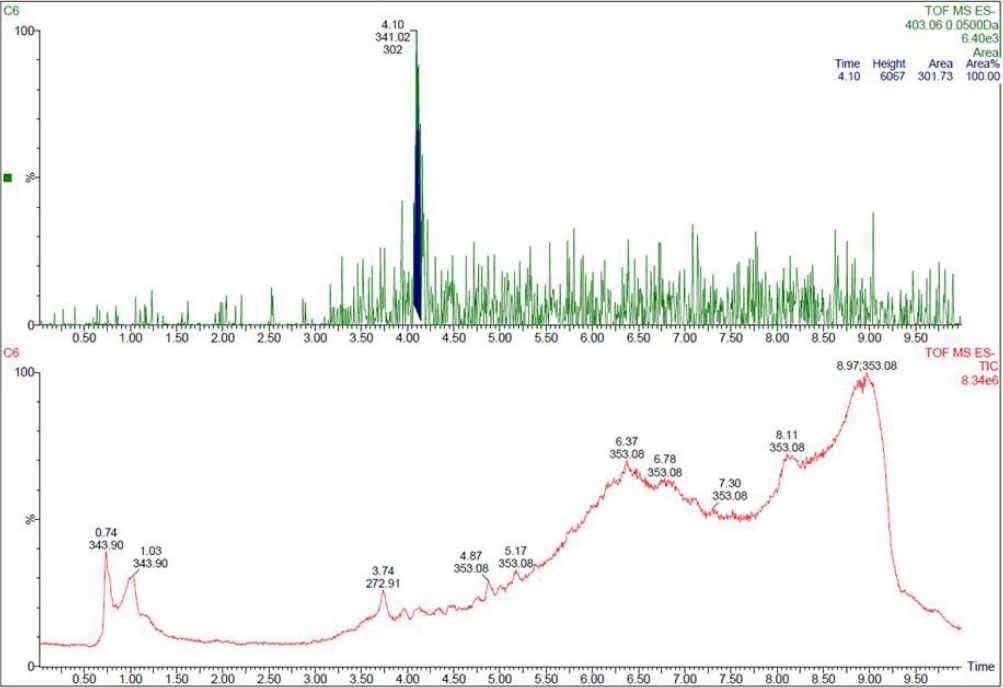
Twelve random urine samples (bottle-fed infants, 9 samples; breastfed infants, 3 samples) were subjected to LCMS/MS for BPA glucuronide. Of these, 9 urine samples (bottle-fed infants, 7; breastfed infants, 2) tested positive for BPA glucuronide by LCMS/MS (Fig. 3). Concentration of BPA glucuronide in bottlefed infants’ urine was 0.95±0.3 ng/mL and in breastfed infants’ urine was 0.8±0.4 ng/mL (P=0.46)
Further, 10 random plasma samples in both groups were subjected to LCMS/MS for BPA glucuronide. None of the plasma samples tested positive for BPA glucuronide.
Breast milk is reported to be best suitable nutrition for an infant and its consumption provides innate immunity to them [31]. This leads to a better ability of the infants to resist the varied infections in life. Studies have reported that formula-fed infants are prone to develop gastrointestinal disturbances, obesity, and type 2 diabetes mellitus in later life [32].
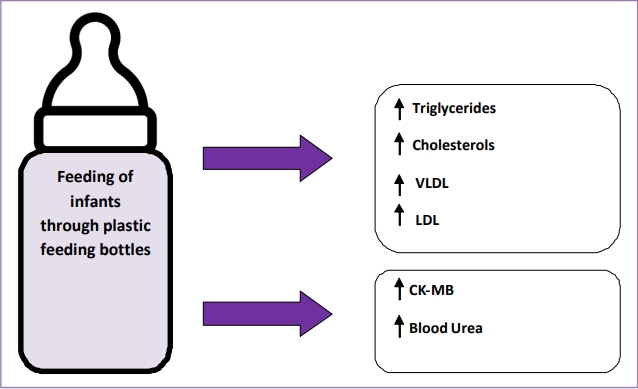
In the present study, the infants who were fed through plastic feeding bottles revealed changes in various biochemical parameters. These alterations manifested in the form of deranged lipid profile in bottle-fed infants as compared to breastfed infants in the present study. Total cholesterol, triglycerides, LDL, VLDL levels were markedly increased in the plastic bottle-fed infants. Further increase in CK-MB and serum urea levels were also recorded in the bottle-fed infants.
Lipid profile derangements are an indicator of cardiovascular injury. CK-MB is produced from cardiac tissue and its increase also marks myocardial damage [33]. Urea is produced in the liver and excreted through kidneys [34]. Any increase in these parameters points towards the imbalance in the function of these vital organs and may also be associated with the increased protein or tissue breakdown inside the body. However, confirmation of the cardiovascular injury is suggested through study of creatinine Kinase, myoglobin, cardiac troponins, and inflammatory markers like C-reactive proteins, Interleukin-6 along with electrocardiographic findings [35].
It may be argued that the aforesaid biochemical changes are the outcome of bovine/goat milk/formula feeding practices. In studies reported elsewhere, the breastfed infants were reported to have high levels of cholesterol, triglycerides, LDL levels as compared to bovine milk-fed infants [36]. However, in the present study, the results are opposite to these studies. These studies however do not mention the use of plastic bottles while feeding the infants. It is pertinent to understand that both bovine milk and breast milk have similar fat content, however, the type of fat differs in both. Bovine milk is rich in saturated fats whereas breast milk is rich in unsaturated fats [37]. This may be an explanation for the difference in both groups for alteration in the lipid profile. However, the increase in CK-MB, serum urea levels reveal that there is something more than only changes in the milk constitution in both groups. The infants of group 2, who were not receiving the maternal feed, were fed through plastic bottles. These infants not only received bovine milk from plastic bottles but also goat milk, water, and top feed. The formula feed may also be considered as a factor behind these findings, but majority of the samples in the present study belonged to infants whose parents belonged to lower socioeconomic status and could not afford costly formula feed every time to feed their infants. Hence it is difficult to say that the biochemical changes are due to the type of food intake alone.
It is reported that plastic bottles leach out BPA or its substitutes like BPAF, BPF, BPS, and phthalates from BPA free plastic bottles [38,39], whenever these bottles are exposed to high temperatures, subjected to cleaning by harsh detergents or change in acidic pH. Feeding through these bottles is expected to expose the infants to these chemicals. This may be a reason for the exposure of the infants to BPA in the present study. According to the history provided by the parents, they cleansed the bottles with detergents and dipped in boiled water before use, which could possibly leach BPA.
Further, BPA glucuronide levels were detected in the urine samples of the infants. Presence of BPA glucuronide in the urine of the infants reveals that the infants were exposed to the chemical. The deranged biochemical parameters may be linked to the presence of BPA, which is probably the leached BPA that had entered the infants’ body. Further, free BPA was detected in one plasma sample of plastic bottle-fed infant. However, the LCMS results also revealed the presence of BPA glucuronide in 2 urine samples of breastfed infants and the result was not significantly different from the bottle-fed infant group. This may be possibly due to the fact that the infants were quite small to show such difference. This makes the involvement of BPA for biochemical changes questionable. The presence of BPA glucuronide in breastfed infants may be attributed to maternal exposure to the chemical which might have been ingested by the infants from the breast milk or possibly contamination of the drinking water with BPA can also be a reason behind the presence of the chemical in the urine samples. But, it is pertinent to note that the biochemical parameters were not affected in breastfed infants. Though samples from the mothers of these infants were not examined, it needs more detailed study to rule out whether this protective action is attributed to the breast milk or the BPA coming in maternal milk or contaminated water is less in quantity and hence is not producing change similar to bottlefed infants. Further, the effects of other chemicals accompanying BPA in plastic bottles like phthalates, BPF, BPS, etc are also expected to contribute to the ill effects and hence the changes are more pronounced in plastic bottle-fed infants. A study on rats from our lab has shown to produce oxidative stress-induced tissue injury due to feeding through plastic bottles [23]. Hence similar tissue changes may be expected in human infants as well.
In the present study, the group of infants who were on plastic bottle feeding had a mixed practice of feeding. Very few mothers fed formula milk and most of them fed bovine milk, goat milk, or water through the plastic bottles to their infants. In a developing country like India, people generally feed bovine milk instead of costly formula feed to their infants in case they are unable to breastfeed their babies. The knowledge about recommended feeding practices is still lacking in majority of women in the country [40-42].
According to Bureau of Indian Standard regulations 2015, the use of BPA is banned in infant feeding bottles, but the manufacturers do not adhere to the guidelines. Further, the measures to check its use in infant bottles are not strictly followed by any agency [43]. A number of studies have shown that BPA leaches out through these bottles which are available in market [44].
In the previous study, infants on bovine milk showed reduced cholesterol, LDL, triglycerides, however, in the present study, the findings were opposite in terms of raised cholesterol, LDL, triglycerides, VLDL in plastic bottle-fed infants. This can be attributed to the usage of plastic bottles to feed the infants which leach out BPA and phthalates, which in turn may alter lipid profile.
The present study had a limitation in terms of sample size. During sample collection, it was noted that the breastfed infants showed fewer OPD visits in comparison to bottle-fed infants and the parents of infants those who visited OPD were reluctant in participating in the study and provide blood or urine samples of their infants. Overall counselling the parents and obtaining samples of infants was a hard task and the sample size in both groups was therefore limited. Further, not all samples could be subjected to LCMS analysis of BPA due to limited funds. In the present study, BPA and BPA glucuronide only were detected; however other endocrine-disrupting agents like BPAF, BPF, BPS, and phthalates were not looked into. Further, evaluation of sex hormones or other environmental hormones/endocrine disruptors was beyond the scope of the present study, but may be looked into in further studies.
Despite these limitations, the present study provides scope for understanding the harmful effects of plastic bottle feeding. Further studies are implicated to look through the effects of plastic bottle feeding in humans. Earlier studies in rodent model have shown biochemical alterations associated with plastic bottle feeding [23]. The possibility of oxidative damage produced by leached BPA from the plastic bottles may be present in human infants as that reported in rats.
In conclusion, the biochemical alterations in the form of deranged lipid profile, raised CK-MB and serum urea levels in plastic bottle-fed infants as compared to breastfed infants along with detection of BPA glucuronide in urine samples reveal the toxic effects of plastic bottle feeding. The endocrine disruptors leached from the plastic bottles may be contributing to such changes.
Acknowledgments
The authors are thankful to UCOST for funding the study. The authors are thankful to Mrs Monika Pandey.
Fig. 1.
Schema for plasma and urine sample collection and use in different tests. LCMS/MS, liquid chromatography-mass spectrometry; BPA, bisphenol A; HPLC, high performance liquid chromatography.
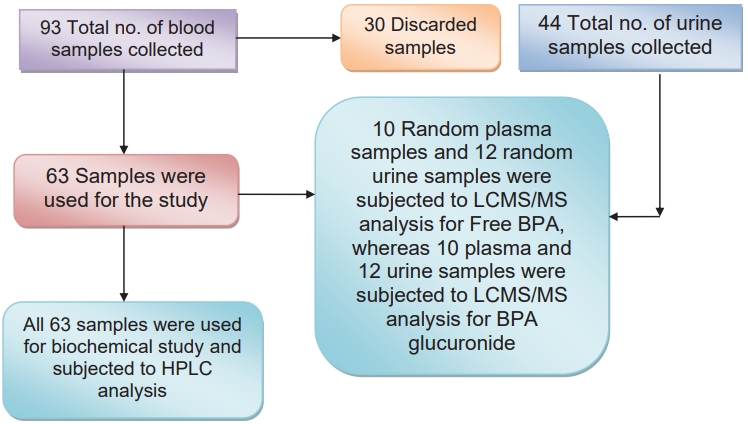
Fig. 2.
Comparison of biochemical parameters between breastfed infants and bottle-fed infants. LDL, lowdensity lipoprotein; VLDL, very low-density lipoprotein; CK-MB, creatine-kinase–MB.
References
1. The 7 types of plastics: their toxicity and what they are most commonly used for [Internet]. Luray (VA): Alan’s Factory Outlet; c2003-2022 [cited 2020 Nov 9]. Available from: https://www.alansfactoryoutlet.com/7-types-of-plastics-their-toxicity-and-most-commonly-used-for.
2. Brede C, Fjeldal P, Skjevrak I, Herikstad H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit Contam 2003;20:684–9.


3. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocr Rev 2009;30:293–342.




4. Kabir ER, Rahman MS, Rahman I. A review on endocrine disruptors and their possible impacts on human health. Environ Toxicol Pharmacol 2015;40:241–58.


5. Cabaton NJ, Wadia PR, Rubin BS, Zalko D, Schaeberle CM, Askenase MH, et al. Perinatal exposure to environmentally relevant levels of bisphenol A decreases fertility and fecundity in CD-1 mice. Environ Health Perspect 2011;119:547–52.



6. Kamel AH, Foaud MA, Moussa HM. The adverse effects of bisphenol A on male albino rats. J Basic Appl Zool 2018;79:6.


7. Smarr MM, Grantz KL, Sundaram R, Maisog JM, Kannan K, Buck Louis GM. Parental urinary biomarkers of preconception exposure to bisphenol A and phthalates in relation to birth outcomes. Environ Health 2015;14:73.




8. Sugiura-Ogasawara M, Ozaki Y, Sonta S, Makino T, Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum Reprod 2005;20:2325–9.


9. Yamasaki K, Sawaki M, Noda S, Imatanaka N, Takatsuki M. Subacute oral toxicity study of ethynylestradiol and bisphenol A, based on the draft protocol for the “Enhanced OECD Test Guideline no. 407”. Arch Toxicol 2002;76:65–74.



10. Brehm E, Flaws JA. Transgenerational effects of endocrine-disrupting chemicals on male and female reproduction. Endocrinology 2019;160:1421–35.




11. Shi M, Sekulovski N, MacLean JA, Whorton A, Hayashi K. Prenatal exposure to bisphenol A analogues on female reproductive functions in mice. Toxicol Sci 2019;168:561–71.


12. Chapin RE, Adams J, Boekelheide K, Gray LE Jr, Hayward SW, Lees PS, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol 2008;83:157–395.


13. Patisaul HB, Fortino AE, Polston EK. Differential disruption of nuclear volume and neuronal phenotype in the preoptic area by neonatal exposure to genistein and bisphenol A. Neurotoxicology 2007;28:1–12.


14. Kiguchi M, Fujita S, Lee J, Shimizu N, Koshikawa N. Behavioral responses to methylphenidate and apomorphine in rats exposed neonatally to bisphenol A. J Oral Sci 2007;49:311–8.


15. Toufexis D. Region and sex specific modulation of anxiety behaviours in the rat. J Neuroendocrinol 2007;19:461–73.


16. Grohs MN, Reynolds JE, Liu J, Martin JW, Pollock T, Lebel C, et al. Prenatal maternal and childhood bisphenol A exposure and brain structure and behavior of young children. Environ Health 2019;18:85.




17. Berger K, Eskenazi B, Balmes J, Kogut K, Holland N, Calafat AM, et al. Prenatal high molecular weight phthalates and bisphenol A, and childhood respiratory and allergic outcomes. Pediatr Allergy Immunol 2019;30:36–46.




18. Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res 2006;66:5624–32.




19. Wetherill YB, Petra CE, Monk KR, Puga A, Knudsen KE. The xenoestrogen bisphenol A induces inappropriate androgen receptor activation and mitogenesis in prostate adenocarcinoma cells. Mol Cancer Ther 2002;1:515–24.

20. Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS. Association of urinary bisphenol A with heart disease: evidence from NHANES 2003/06. PLoS One 2010;5:e8673.



21. Braun JM, Li N, Arbuckle TE, Dodds L, Massarelli I, Fraser WD, et al. Association between gestational urinary bisphenol A concentrations and adiposity in young children: the MIREC study. Environ Res 2019;172:454–61.



22. Hoepner LA. Bisphenol A: a narrative review of prenatal exposure effects on adipogenesis and childhood obesity via peroxisome proliferatoractivated receptor gamma. Environ Res 2019;173:54–68.



23. Pant MK, Ahmad AH, Naithani M, Pandey HS, Pandey M, Pant J. Effect of exposure of plastic infant feeding bottle leached water on biochemical, morphological and oxidative stress parameters in rats. Toxics 2020;8:34.



24. Almeida S, Raposo A, Almeida-González M, Carrascosa C. Bisphenol A: food exposure and impact on human health. Compr Rev Food Sci Food Saf 2018;17:1503–17.



25. Lehmler HJ, Liu B, Gadogbe M, Bao W. Exposure to bisphenol A, bisphenol F, and bisphenol S in U.S. adults and children: the National Health and Nutrition Examination Survey 2013–2014. ACS Omega 2018;3:6523–32.




26. Carwile JL, Luu HT, Bassett LS, Driscoll DA, Yuan C, Chang JY, et al. Polycarbonate bottle use and urinary bisphenol A concentrations. Environ Health Perspect 2009;117:1368–72.



27. Nahar MS, Liao C, Kannan K, Dolinoy DC. Fetal liver bisphenol A concentrations and biotransformation gene expression reveal variable exposure and reduced capacity for metabolism in humans. J Biochem Mol Toxicol 2013;27:116–23.



28. Baluka SA, Rumbeiha WK. Bisphenol A and food safety: lessons from developed to developing countries. Food Chem Toxicol 2016;92:58–63.


29. Bittner GD, Yang CZ, Stoner MA. Estrogenic chemicals often leach from BPA-free plastic products that are replacements for BPA-containing polycarbonate products. Environ Health 2014;13:41.




32. Stuebe A. The risks of not breast feeding for mothers and infants. Rev Obstet Gynecol 2009;2:222–31.


33. Carvalho G, Rassi S. The prognostic value of CK-MB in acute myocardial infarction in developing countries: a descriptive study. Angiology 2016;4:1000183.

34. Higgins C. Urea and the clinical value measuring blood urea concentration [Internet]. Brønshøj (Denmark): Radiometer Medical ApS; c2018 [cited 2020 Nov 9]. Available from: https://acutecaretesting.org/en/articles/urea-and-the-clinical-value-of-measuring-blood-urea-concentration.
36. Harit D, Faridi MMA, Aggarwal A, Sharma SB. Lipid profile of term infants on exclusive breastfeeding and mixed feeding: a comparative study. Eur J Clin Nutr 2008;62:203–9.



37. A comparison between human milk and cow’s milk [Internet]. Bristol (UK): Viva; c2022 [cited on 2020 Nov 9] Available from: https://www.viva.org.uk/white-lies/comparison-between-human-milk-and-cows-milk.
38. Thoene M, Dzika E, Gonkowski S, Wojtkiewicz J. Bisphenol S in food causes hormonal and obesogenic effects comparable to or worse than bisphenol A: a literature review. Nutrients 2020;12:532.



39. Bottles Can be Toxic—Part, II [Internet]. New Delhi (India): Toxicslink.org; c1992-2022 [cited 2020 Apr 25] Available from http://www.toxicslink.org/?q=content/bottles-can-be-toxicpart-ii.
40. Mayuri M, Garg V, Mukherji C, Aggarwal D, Ganguly S. Bovine milk usage and feeding practices for infants in India. Indian J Public Health 2012;56:75–81.


41. Mahmood SE, Srivastava A, Shrotriya VP, Mishra P. Infant feeding practices in the rural population of north India. J Family Community Med 2012;19:130–5.



42. Mehlawat U, Puri S, Rekhi TK, Yadav BS, Tiwari SK. A study on infant and young child feeding practices of mothers visiting District Civil Hospital. New Indian J Pediatr 2018;7:178–88.

43. Indian Standard PLASTICS FEEDING BOTTLES (First Revision) [Internet]. New Delhi (India): Bureau of Indian Standards; 2015 [cited 2022 Mar 16]. Available from: http://bpni.org/IMS-ACT/REVISED-FEEDING-BOTTLE-STANDARDS-2015.pdf.
44. Traces of banned bisphenol A found in baby bottles. DownToEarth [Internet]. 2019 May 6 [cited 2022 Mar 16]]; Health. Available from: https://www.downtoearth.org.in/news/health/traces-of-banned-bisphenol-a-found-in-baby-bottles-64344.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


